Science & Pipeline

Science'r'us
BRP is providing novel approaches for dermatology treatments based on sound scientific discoveries. With patented and scientifically proven discoveries, we are targeting unmet medical niches with significant patient populations.
Latanoprost is used in the treatment of glaucoma and has also been found to stimulate hair growth, particularly in inducing hypertrichosis of the eyelashes among patients using latanoprost eye drops. However, its high skin penetration raises concerns about potential systemic adverse events. BRP-011, a prostaglandin analogue, has emerged as an alternative key component. It was initially dismissed as a potential topical treatment due to its low bioavailability.
BRP’s discovery has revealed the efficacy of asset in significantly promoting hair growth as demonstrated in studies involving individuals with androgenetic alopecia. These promising results have led to the filing of a patent. Given the vast market for androgenetic alopecia, with over 200 million patients falling within the patent-protected domain, BRP-011 is well positioned for potential commercialization success, as confirmed by a favorable credible external assessment.
We have discovered that BRP-007, in addition to its antimicrobial activity, is also a potent anti-inflammatory agent. It acts as an inhibitor of receptors for pro-inflammatory factors – cytokines from the common gamma chain group.
We demonstrated that use of BRP-007 resulted in improvement in patients with psoriasis.
These discoveries are patented in the US, EU and Australia for use in psoriasis. Psoriasis represents a significant market, with up to 3% of the population translating into over 10 million patients in the patent-protected area (IQVIA report data on file). BRP-007 with its unique mechanism of action, holds blockbuster potential, as confirmed by independent research.
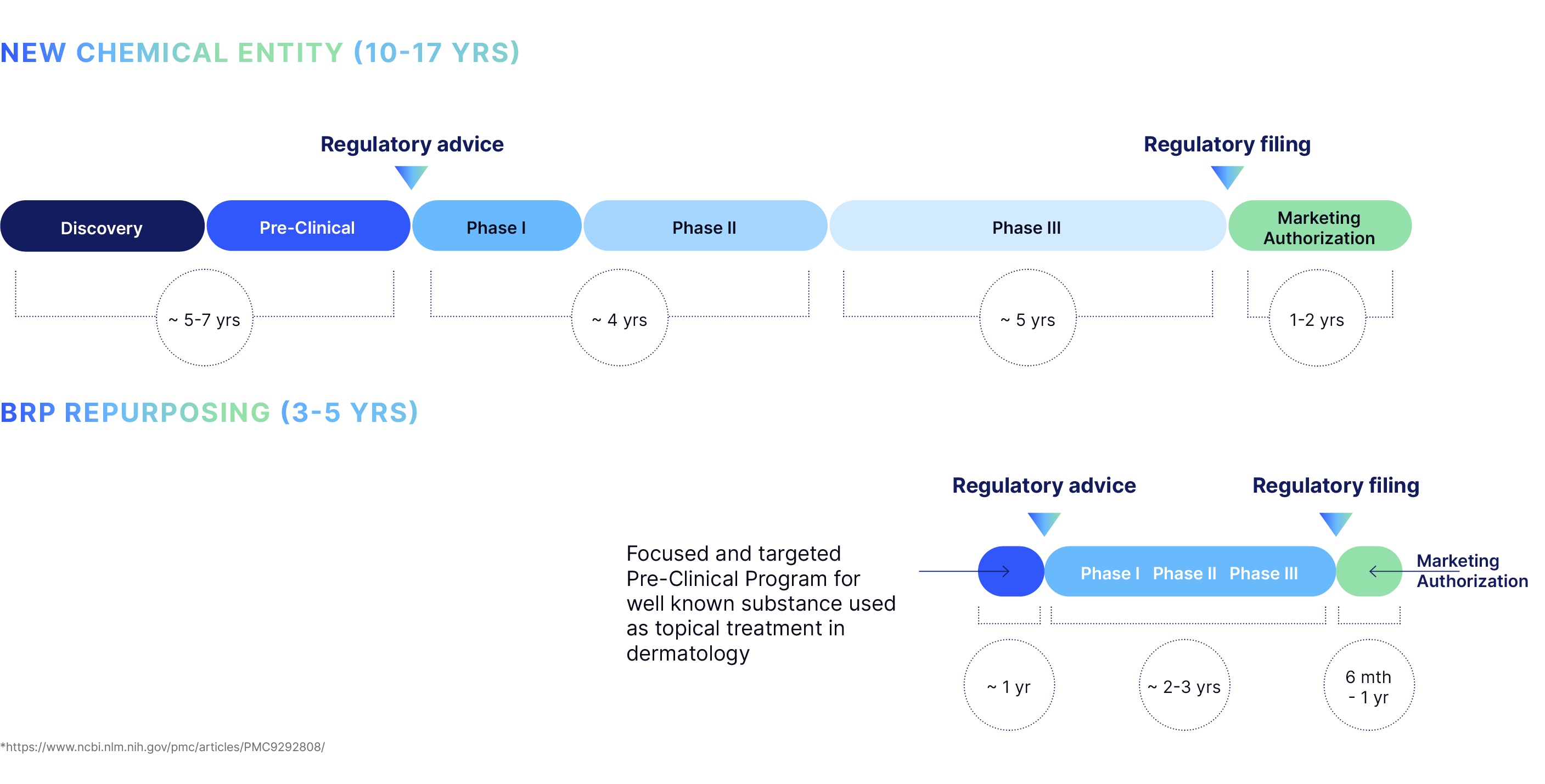
BRP-011 and BRP-007 are at the same stage of the drug development process.

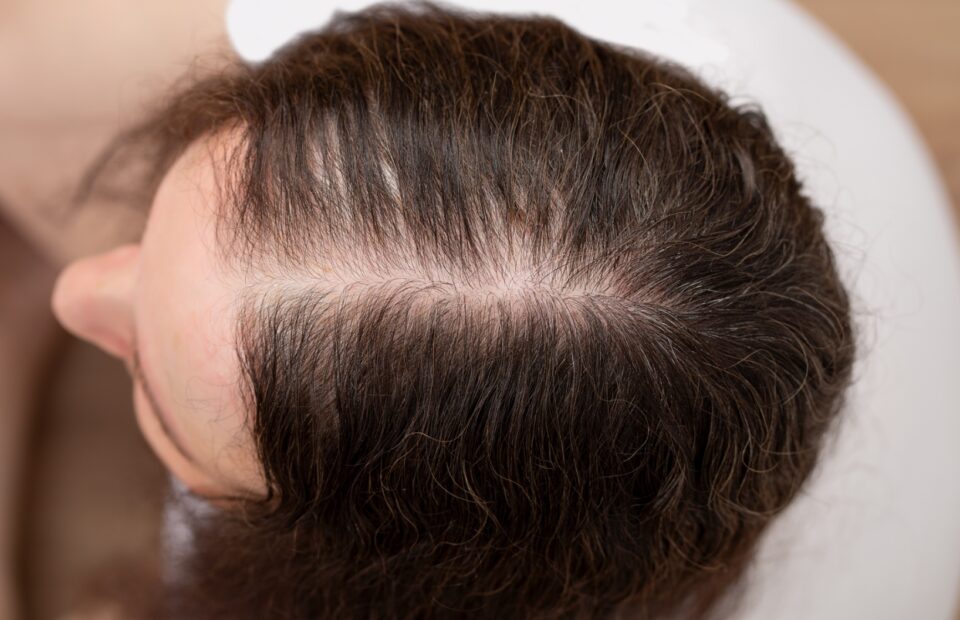
Androgenetic alopecia is a genetic condition characterized by the progressive miniaturization of hair follicles, leading to hair thinning and eventual hair loss. As a common age-dependent hair-loss disorder, it affects up to 70% of men and 40% of women. Unsatisfactory effectiveness and side effect profiles limit current treatment options. There are currently only two drugs approved, with no new registrations for over 20 years. (IQVIA report data on file). The discovery of a fundamental role of prostaglandins in modulating hair function provided novel insights and approaches to drug development. BRP-011 is an active metabolite of latanoprost (stronger agonist of PGFα receptor than latanoprost), and therefore a potent activator of prostaglandin-dependent hair growth. As a monotherapy or in combination with existing therapies, BRP-011 holds the potential to become the cure for androgenetic alopecia in both men and women. This has been clearly confirmed by the positive IQVIA assessment.
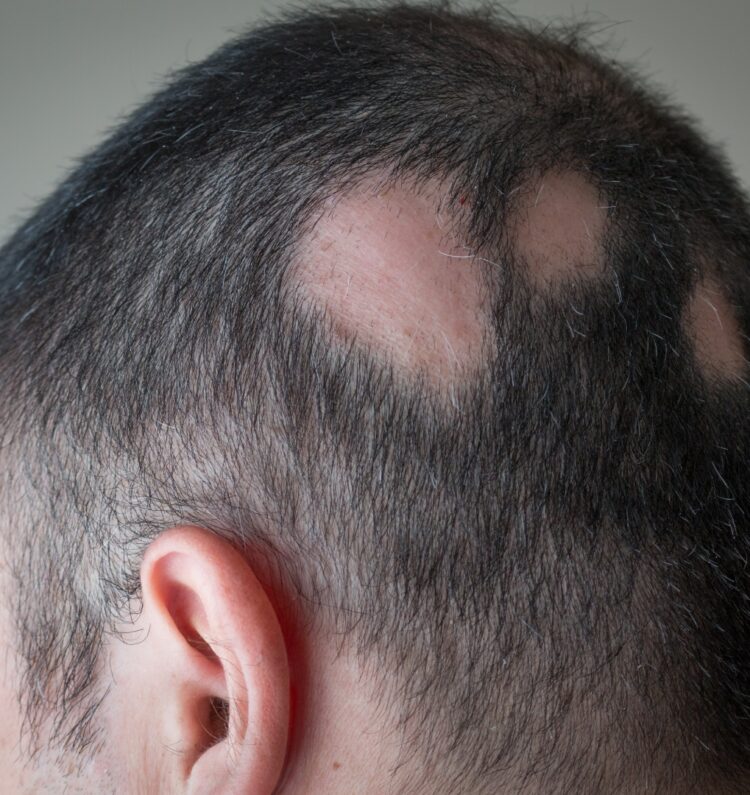
Alopecia areata is an autoimmune disorder that causes hair loss in patches on the scalp and sometimes other areas of the body. It occurs when the immune system mistakenly attacks the hair follicles, leading to hair loss. This condition can affect individuals of all ages and often begins during childhood or young adulthood. The patches of hair loss can be small or large and may come and go, making the hair loss unpredictable. While the exact cause is unknown, genetics and environmental factors are believed to play a role. There is no known cure for alopecia areata, but treatments like corticosteroid injections, topical corticosteroids, and immunotherapy can help stimulate hair regrowth in some cases.
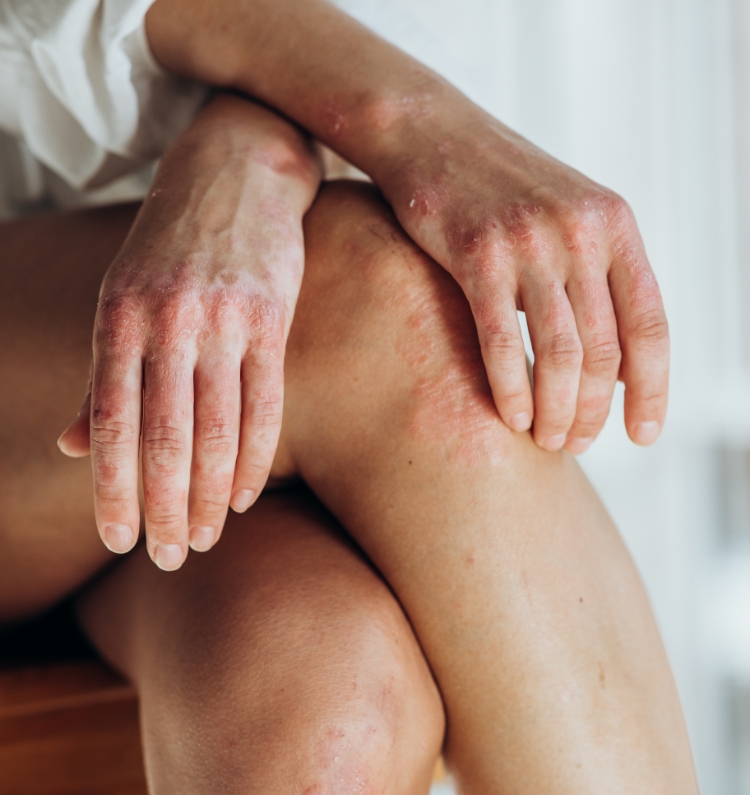
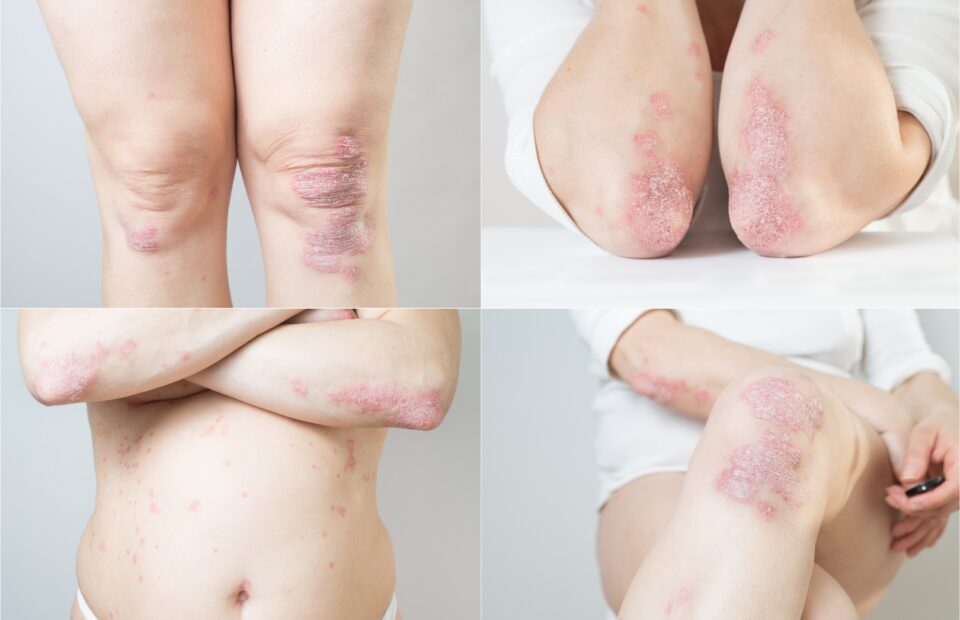
Psoriasis is one of the most common chronic inflammatory skin diseases characterized by scaly and itchy, sometimes painful, exudated and bleeding skin lesions. The etiopathogenesis of psoriasis is complex and still not fully understood. The advent of biologic therapy has significantly improved the management of psoriasis, but there is still a need for new effective topical drugs.
At the foundation of BRP-007 development is our discovery of asset's previously unknown ability to block multiple pro-inflammatory cytokines.
By blocking the important inflammatory pathways, BRP-007 inhibits the cascade of biological reactions that trigger development of psoriatic lesions. Due to its novel and unique mode of action, BRP-007 holds the potential to become a safe and efficacious topical treatment for psoriasis and atopic dermatitis - used alone or in combination with other treatments as confirmed by IQVIA analysis and positive assessment.
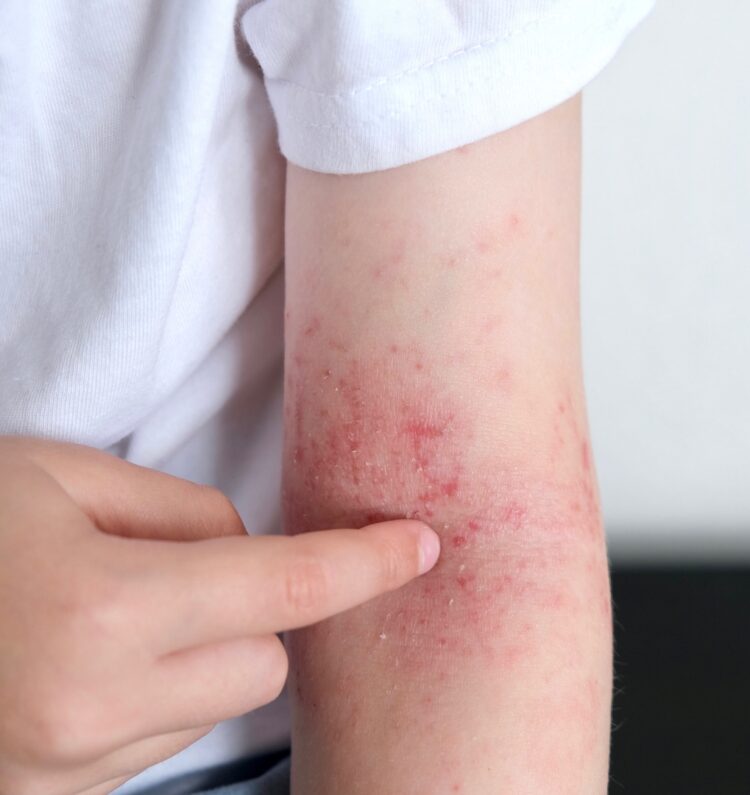
Atopic dermatitis is a chronic skin condition characterized by red, itchy, and inflamed patches of skin. It typically begins in childhood and may persist into adulthood. These patches can appear anywhere on the body and are often accompanied by dry, scaly skin. The exact cause of atopic dermatitis is unknown, but it is believed to involve a combination of genetic, environmental, and immune system factors. Triggers such as allergens, stress, irritants, and weather changes can exacerbate symptoms. While there's no cure for atopic dermatitis, various treatments like moisturizers, topical corticosteroids, antihistamines, and lifestyle adjustments can help manage symptoms and provide relief.